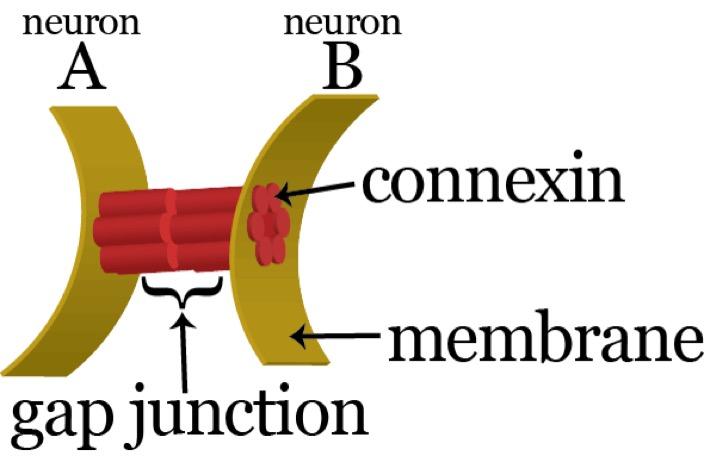
We are interested in unraveling the fundamental principles of brain operation. What are the basic features that any brain of any animal should possess in order to produce coherent and adaptive behavior? How do brains implement these essential functions as an integral system? What is the role of neuronal connectivity in determining brain function?
We use two complementary approaches to address these questions. (1) Analysis of existing basic brain functions and circuits, examining how they co-function and co-exist. This provides fundamental knoweldge about system-level coordination and integration. (2) Synthesis of new synaptic connections and, ultimately, of entire new brain circuits, to achieve a construction-based level of understanding, and uncover the causative role of connectivity in circuit function. We conduct both of these forms of research using the relatively simple and well-characterized nervous system of the miniature roundworm C. elegans. C. elegans enables us to reduce complex problems into much simpler ones, in which the essential principles of brain operation can be traced. It also serves as a powerful platform for genetic engineering of new synaptic connections and circuit reconfiguration.