H. S. Hahm, M. K. Schlegel, M. Hurevich, S. Eller, F. Schuhmacher, J. Hofmann, K. Pagel, and P. H. Seeberger. 2017. “
Automated glycan assembly using the Glyconeer 2.1 synthesizer.” Proceedings of the National Academy of Sciences of the United States of America, 114, 17, Pp. E3385-E3389.
Abstract Reliable and rapid access to defined biopolymers by automated DNA and peptide synthesis has fundamentally altered biological research and medical practice. Similarly, the procurement of defined glycans is key to establishing structure–activity relationships and thereby progress in the glycosciences. Here, we describe the rapid assembly of oligosaccharides using the commercially available Glyconeer 2.1 automated glycan synthesizer, monosaccharide building blocks, and a linker-functionalized polystyrene solid support. Purification and quality-control protocols for the oligosaccharide products have been standardized. Synthetic glycans prepared in this way are useful reagents as the basis for glycan arrays, diagnostics, and carbohydrate-based vaccines.

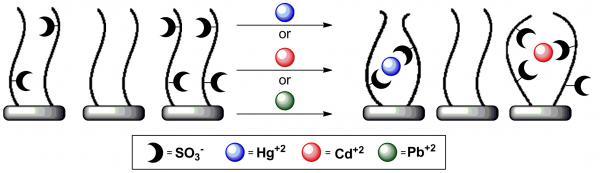
E. Mervinetsky, I. Alshanski, Y. Hamo, L. M. Sandonas, A. Dianat, J. Buchwald, R. Gutierrez, G. Cuniberti, M. Hurevich, and S. Yitzchaik. 2017. “
Copper Induced Conformational Changes of Tripeptide Monolayer Based Impedimetric Biosensor.” Scientific Reports, 7.
Abstract Copper ions play a major role in biological processes. Abnormal Cu2+ ions concentrations are associated with various diseases, hence, can be used as diagnostic target. Monitoring copper ion is currently performed by non-portable, expensive and complicated to use equipment. We present a label free and a highly sensitive electrochemical ion-detecting biosensor based on a Gly-Gly-His tripeptide layer that chelate with Cu2+ ions. The proposed sensing mechanism is that the chelation results in conformational changes in the peptide that forms a denser insulating layer that prevents RedOx species transfer to the surface. This chelation event was monitored using various electrochemical methods and surface chemistry analysis and supported by theoretical calculations. We propose a highly sensitive ion-detection biosensor that can detect Cu2+ ions in the pM range with high SNR parameter.

Several multistep strategies were developed to ensure single methylation of amines on solid support. These strategies rely on the introduction of the o-NBS protecting/activating group as a key step. We found that the state-of-the-art strategies fail for the methylation of several primary amine motifs, largely due to inefficient sulfonylation. Here we show that using the superior nucleophilic base DMAP instead of the commonly used base collidine as a sulfonylation additive is essential for the introduction of the o-NBS group to these amine motifs. DFT calculations provide an explanation by showing that the energy barrier of the DMAP intermediate is significantly lower than the one of the collidine. We demonstrate that using DMAP as a sole additive in the sulfonylation step results in an overall effective and regioselective N-methylation. The method presented herein proved highly efficient in solid-phase synthesis of a somatostatin analogue bearing three N α-methylation sites that could not be synthesized using the previously described state-of-the-art methods.
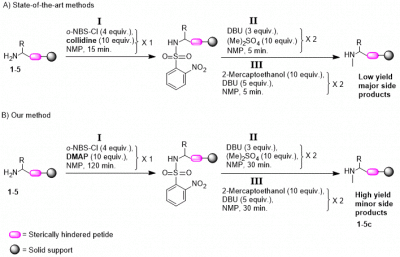
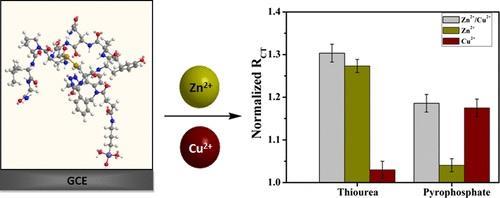
Kiran Kumar Tadi, Israel Alshanski, Evgeniy Mervinetsky, Gerard Marx, Panayiota Petrou, Karussis M. Dimitrios, Chaim Gilon, Mattan Hurevich, and Shlomo Yitzchaik. 2017. “
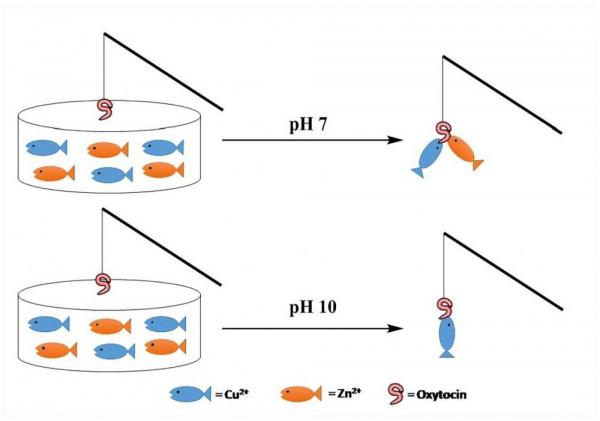
Oxytocin-Monolayer-Based Impedimetric Biosensor for Zinc and Copper Ions.” ACS Omega, 2, 12, Pp. 8770-8778.
Abstract Zinc and copper are essential metal ions for numerous biological processes. Their levels are tightly maintained in all body organs. Impairment of the Zn2+ to Cu2+ ratio in serum was found to correlate with many disease states, including immunological and inflammatory disorders. Oxytocin (OT) is a neuropeptide, and its activity is modulated by zinc and copper ion binding. Harnessing the intrinsic properties of OT is one of the attractive ways to develop valuable metal ion sensors. Here, we report for the first time an OT-based metal ion sensor prepared by immobilizing the neuropeptide onto a glassy carbon electrode. The developed impedimetric biosensor was ultrasensitive to Zn2+ and Cu2+ ions at physiological pH and not to other biologically relevant ions. Interestingly, the electrochemical impedance signal of two hemicircle systems was recorded after the attachment of OT to the surface. These two semicircles suggest two capacitive regions that result from two different domains in the OT monolayer. Moreover, the change in the charge-transfer resistance of either Zn2+ or Cu2+ was not similar in response to binding. This suggests that the metal-dependent conformational changes of OT can be translated to distinct impedimetric data. Selective masking of Zn2+ and Cu2+ was used to allow for the simultaneous determination of zinc to copper ions ratio by the OT sensor. The OT sensor was able to distinguish between healthy control and multiple sclerosis patients diluted sera samples by determining the Zn/Cu ratio similar to the state-of-the-art techniques. The OT sensor presented herein is likely to have numerous applications in biomedical research and pave the way to other types of neuropeptide-derived sensors.