Citation:
E Vaganova, M Rozenberg, F Dubnikova, D Danovich, and S Yitzchaik. 2015. “Acidity of the Methyne Group of poly(4-vinylpyridine) Lead to Side-Chain Protonation in Pyridine.” New J.Chem, 39, 8, Pp. 5920-5922. Article
Abstract:
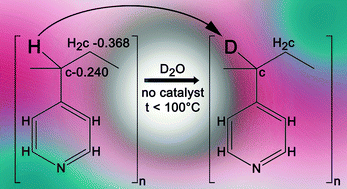
Poly(4-vinylpyridine) swollen in pyridine displays changes in electrical conductivity in response to white light and to low level thermal perturbation; protonation of the side-chain nitrogen is believed to play a role. Here we present spectroscopic evidence that the proton donor is the methyne group CH on the polymer chain.