Citation:
Date Published:
2020Abstract:
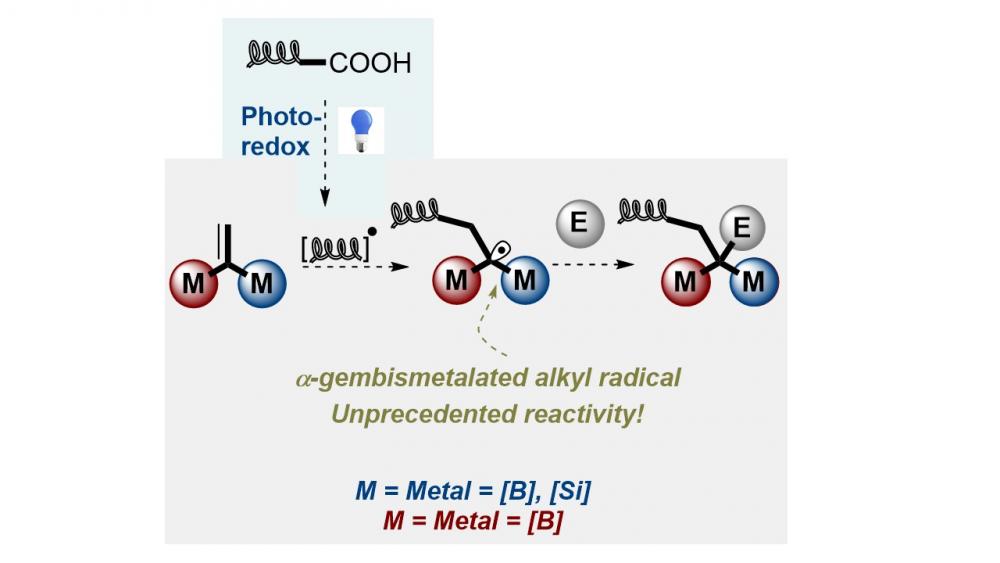
The use of gem-diborylalkenes as light-reactive groups is explored for the first time. These reactions provide an efficient and general method for the photochemical conversion of gem-diborylalkenes to rapidly access 1,1-bisborylalkanes. This method exploits a novel photoredox decarboxylative radical addition to gem-diborylalkenes to afford α-gemdiboryl carbon-centered radicals, which benefit from additional stability by virtue of an interaction with the empty p-orbitals on borons. The reaction offers a highly modular and regioselective approach to γ-amino gem-diborylalkanes. Furthermore, EPR spectroscopy and DFT calculations have provided insight into the radical mechanism underlying the photochemistry reaction.