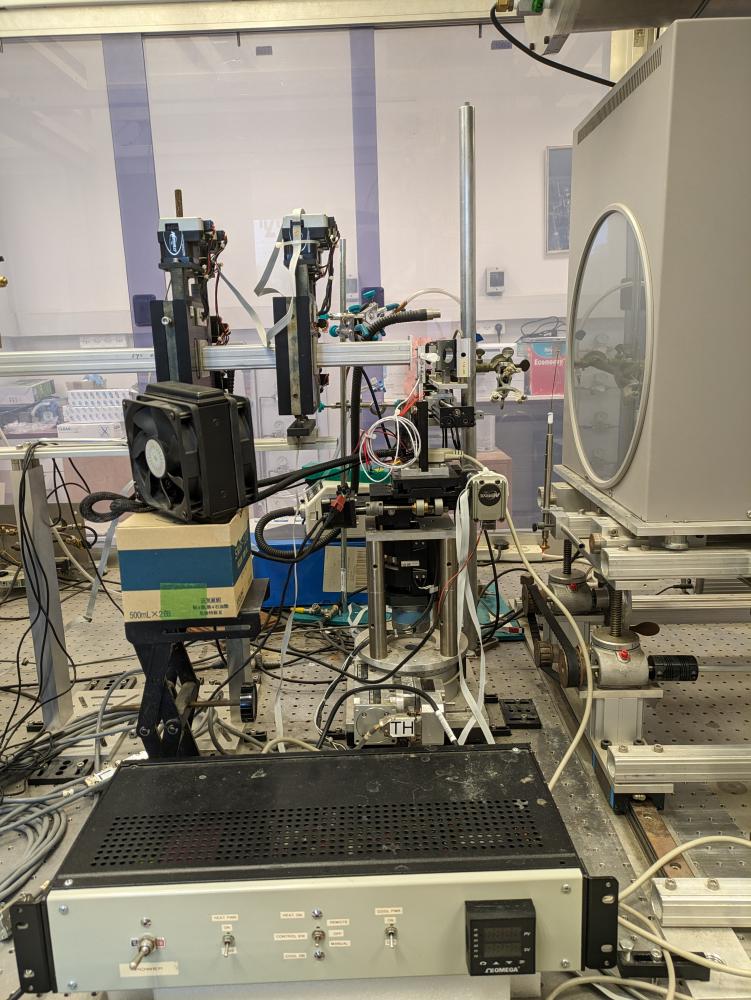
Our lab is equipped with a state-of-the-art small-angle and wide-angle X-ray scattering (SAXS/WAXS) set-up, covering spatial correlation distances between 0.2 and 100 nm. This setup was designed and built in our lab and is one of the best in-house setups (see Soft Matter 2011). Since Oct 2008 high quality data are obtained in our lab that are equivalent to second generation (bending magnet) synchrotron sources.
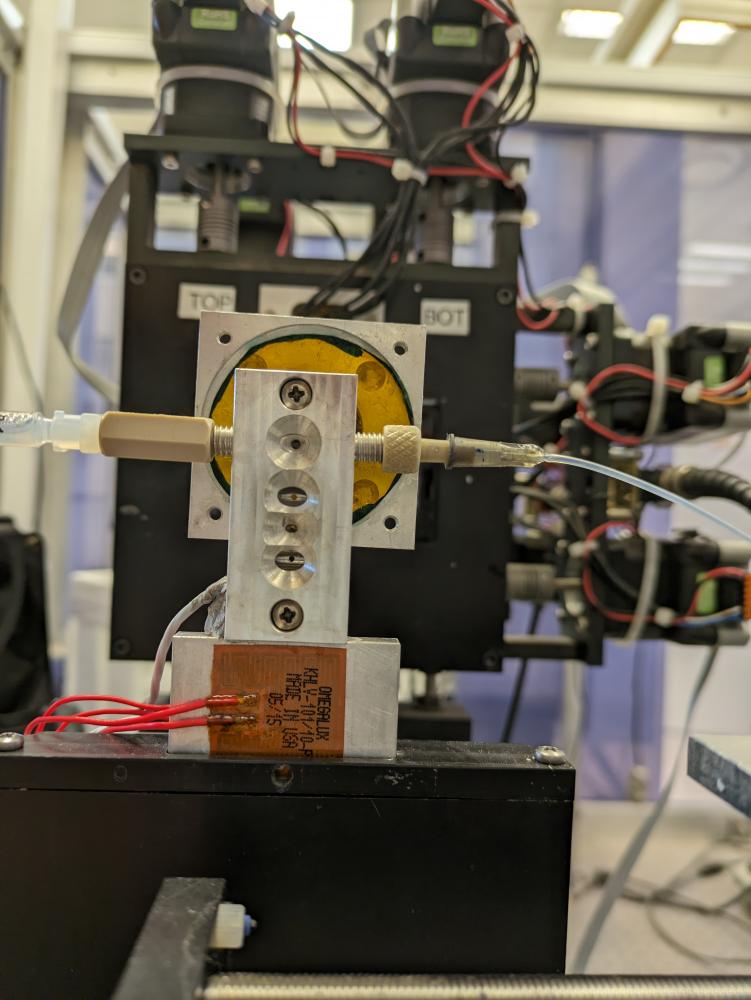
More recently we developped a temperature controlled flow-through capillary setup.
In addition, we are developing state-of-the-art data analysis software for modeling and fitting solution X-ray scattering data. Using geometric models and/or atomic models of subunits and hierarchical modeling approach, any complex molecular structure or architecture can be modeled and compared with solution X-ray scattering data. Our software can integrated with simulations (Biophys. J 2017), numerical solutions of the Poisson–Boltzmann equation (Soft Matter 2013, Langmuir 2017), and theoretical models (ACS Nano 2017) and various algorithms and computational approaches and optimization algorithms (Journal of Parallel and Distributed Computing 2016), like maximum entropy. The latter approaches are useful when we need to resolve the kinetics and thermodynamics of ensembles of dynamic structures.
Time-resolved SAXS experiments, using stopped-flow setups are done at synchrotron facilities (ID02, P12, SWIMG beamlines). Size exchlusion chromatography (SEC) is used at all the synchrotron facilities to isolate specific structures or subunits. in-line SEC-SAXS experiments are done at P12 and SWIMG beamlines.
In addition, we have a light microscopy imaging lab containing high quality differential interference contrast (DIC), polarized and confocal microscopes. We have a biochemical lab that includes a nano-drop and temperature controlled UV-VIS spectrophotometer, two osmometers, refractometer, demistometer, dynamic light scattering (DLS), and more. We are using cryo-TEM (in collaboration with Dr. Yael Levi-Kalisman) through the Nanocenter of the Hebrew University and protein purifiaction equipment in the protein purification facility units of the Hebrew University.
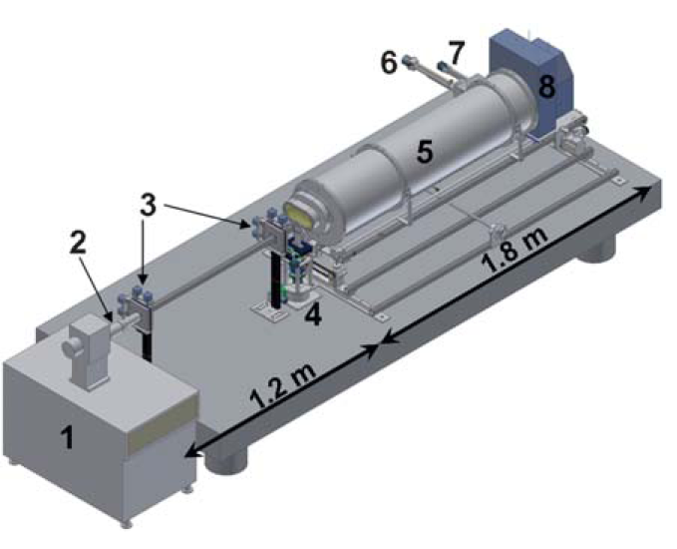
X-Ray scattering setup: (1) MicroMax-007 HF microfocus X-ray generator, (2) Confocal Max-Flux optics, (3) scatterless slits, (4) motorized sample-stage (X–Y–Z and θ), (5) He filled flight-path on a motorized stage, (6) motorized sample insertion in-line Wide Angle X-ray Scattering (WAXS) holder, (7) motorized beam-stop, and (8) MAR345 Image Plate Detector. The source to sample distance is ca. 1.2 metre and the sample to detector distance is ca. 1.8 metre.