We are studying the potential use of ionic liquids as smart alternative solvents that will act as novel molecular chaperons to optimize and direct molecular self assembly into well-ordered structures. While there has been persistent activity directed towards tailoring of self-assembly blocks in order to get supramolecular structures of desired complexity, we address the problem of tailored self-assembly from the opposite direction: we vary, modify and tailor the properties of the solvent itself in order to facilitate and direct self-assembly. We therefore studied the self-assembly of biomolecules in ionic liquids and focused on lipid bilayers (Soft Matter 2013, Langmuir 2014).
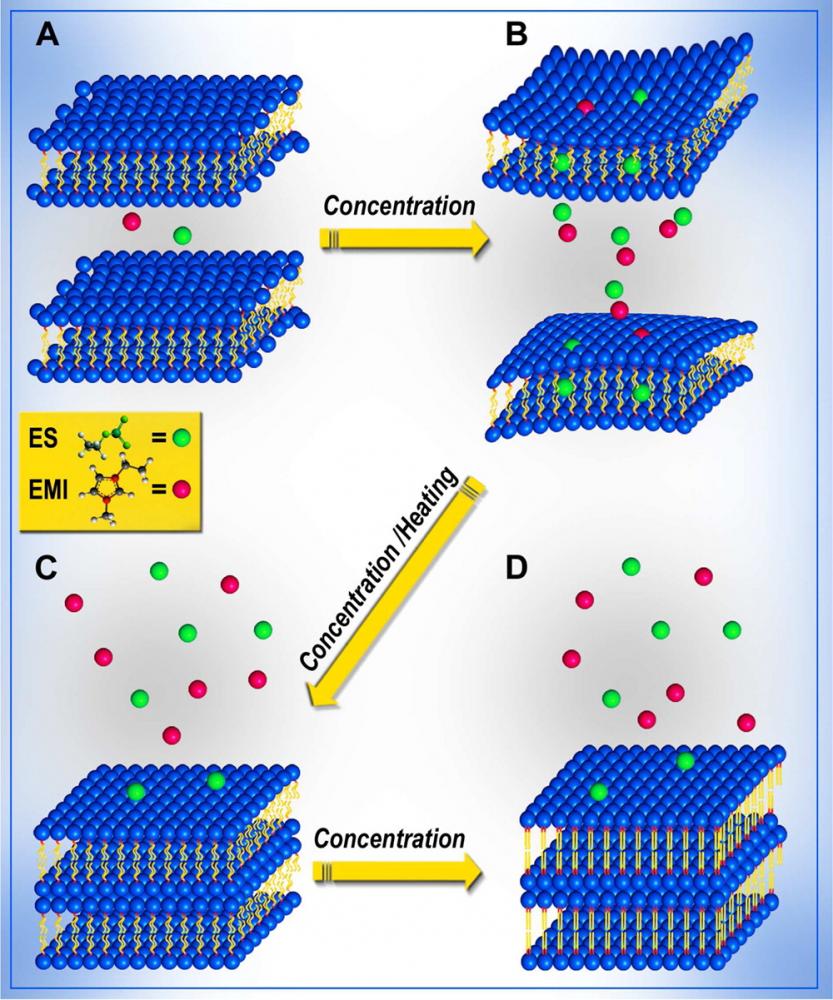
In the future we will extend this to other asseblies that cannot form stable structures in aqueous solution, however, after equilibrating these structures in ionic liquids we will try to transfer back those structures gradually into aqueous solutions, using dialysis methods. If those structures reach an optimal assembly in the ionic liquid environment, they may remain stable also when transferred back to aqueous solvents. Understanding those processes may create, in the future, new crystallization methods for membrane proteins, extended \(\beta\)-sheet forming peptides (e.g. amyloidic peptides) and large protein complexes that still pose a major scientific challenge.
Using a surface force balance with extreme sensitivity and resolution, we have studied directly and quantitatively the lateral and normal interactions as a function of the separation distance between atomically-smooth negatively-charged solid mica surfaces immersed in aqueous solutions. We have found that, under conditions of extreme confinement, pure water and dilute salt solutions retain their bulk fluidity even when confined to films in the thickness range 3.5±1 to 0.0±0.4 nm (Nature 2001). This is unlike the behavior of non-associative solvents (whose viscosity diverges when confined to similarly thin films) and attributed to the fact that confinement suppresses the formation of the highly directional hydrogen-bonded network associated with freezing of water. As a result, pure water and dilute salt solutions confined between mica surfaces are completely squeezed out with ease. At higher salt concentrations, the surface-attached hydration layers keep the compressed surfaces apart. This is owing to strongly-repulsive hydration forces originating in the tenacity of the bound water molecules. Nevertheless we showed that the confined films retain a shear fluidity characteristic of the bulk liquid, even when compressed, at pressures up to ca. 10 atmospheres, down to films 1.0±0.3 nm thick (Science 2002). We attributed this to the ready exchange (as opposed to loss) of water molecules within the hydration layers as they slide past each other. This combination leads to the hydrated layers acting as very efficient lubricants (see relevant publicaitons).


