Citation:
Abstract:
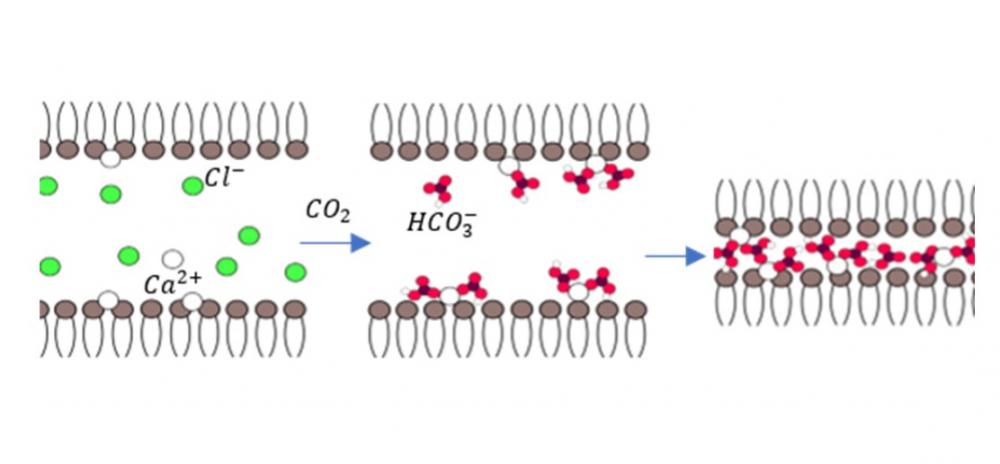
The interaction between lipid membranes and ions is associated with a range of key physiological processes. Most earlier studies have focused on the interaction of lipids with cations, while the specific effects of the anions have been largely overlooked. Owing to dissolved atmospheric carbon dioxide, bicarbonate is an important ubiquitous anion in aqueous media. In this paper, we examined the effect of bicarbonate anions on the interactions between dipolar lipid membranes in the presence of previously adsorbed calcium cations. Using a combination of solution X-ray scattering, osmotic stress, and molecular dynamic simulations, we followed the interactions between 1,2-didodecanoyl-sn-glycero-3-phosphocholine (DLPC) lipid membranes that were dialyzed against CaCl2 solutions in the presence and absence of bicarbonate anions. Calcium cations adsorbed onto DLPC membranes charge them and lead to their swelling. In the presence of bicarbonate anions, however, the calcium cations can tightly couple one dipolar DLPC membrane to the other and form a highly condensed and dehydrated lamellar phase with a repeat distance of 3.45±0.02 nm. Similar tight condensation and dehydration has only been observed between charged membranes in the presence of multivalent counterions. Bridging between bilayers by calcium bicarbonate complexes induced this arrangement. Furthermore, in this condensed phase the lipid molecules and the adsorbed ions were arranged in a 2D oblique lattice.
Notes: