Citation:
Abstract:
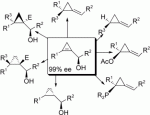
The copper-catalyzed carbomagnesiation (or hydrometalation) reaction of chiral cyclopropenylcarbinol derivatives, obtained by means of a kinetic resolution of secondary allylic alcohols, leads to an easy and straightforward preparation of enantiomerically pure alkylidenecyclopropane derivatives. The reaction mechanism is composed of a syn-carbometalation followed by a syn-elimination reaction. To gain further insight into the reaction mechanism of the carbometalation, the diastereoselective formation of cyclopropylcarbinol was also achieved and was found to be very sensitive to the nature of the organometallic species used for the addition reaction. Cyclopropylcarbinol could also be prepared through a diastereoselective reduction of cyclopropenylcarbinol derivatives. Finally, functionalization of enantiomerically enriched cyclopropenylcarbinols into the corresponding acetate or phosphinite derivatives leads, under mild conditions, to various enantiomerically pure heterosubstituted alkylidenecyclopropanes.

