Citation:
Masarwa, A. ; Stanger, A. ; Marek*, I. An Efficient , Facial, and General Stereoselective Synthesis of Heterosubstituted Alkylidenecyclopropanes. Angew. Chem., Int. Ed. 2007, 46, 8039-8042.
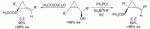
Abstract:
With just a nudge (in the form of silica gel, an acidic ion-exchange resin, or heating at about 40 °C), the acetate derivatives of enantiomerically enriched cyclopropenyl alcohols undergo sigmatropic rearrangement to give alkylidenecyclopropanes with high ee values (see scheme). Similarly, the rearrangement of phosphinite derivatives at room temperature leads to phosphine oxide precursors of unusual chiral phosphine ligands. R1, R2=alkyl, aryl.

