Citation:
Abstract:
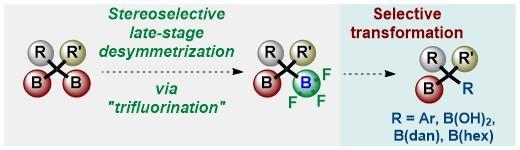
An efficient and general method for the chemoselective synthesis of unsymmetrical gem-diborylalkanes is reported. This method is based on a novel late-stage desymmetrization via nucleophilic “trifluorination”, providing chiral gem-diborylalkanes bearing a trifluoroborate group. The reaction offers a highly modular and diastereoselective approach to synthesize gem-diborylcyclopropanes. The utility of the gem-diborylalkane building blocks was demonstrated by selective post-functionalization of the trifluoroborate group. These functionalizations include inter- and intra- Pd-catalyzed Suzuki−Miyaura coupling reactions.
Notes:
Selected as Hot Paper
Has been recognized as one of the most read in Chemistry - A European Journal for the years 2018-2019!

