Citation:
Abstract:
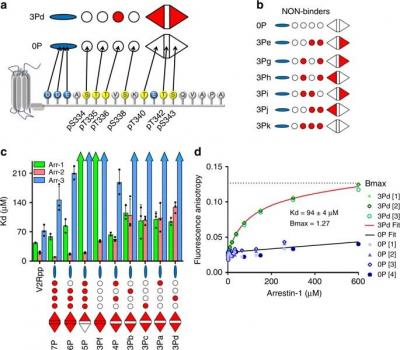
The cellular functions of arrestins are determined in part by the pattern of phosphorylation on the G protein-coupled receptors (GPCRs) to which arrestins bind. Despite high-resolution structural data of arrestins bound to phosphorylated receptor C-termini, the functional role of each phosphorylation site remains obscure. Here, we employed a library of synthetic phosphopeptide analogues of the GPCR rhodopsin C-terminus and determined the ability of these peptides to bind and activate arrestins using a variety of biochemical and biophysical methods. We further characterized how these peptides modulated the conformation of arrestin-1 by nuclear magnetic resonance (NMR). Our results indicate that the particular phosphorylation sites can be grouped into different functional classes: ‘key sites’ required for arrestin binding and activation, an ‘inhibitory site’ that abrogates arrestin binding, and ‘modulator sites’ that influence the global conformation of arrestin. These functional motifs allow a better understanding of how different GPCR phosphorylation patterns might control how arrestin functions in the cell.