Citation:
Abstract:
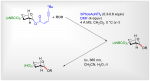
Main observation and conclusion A green and efficient photolabile protecting group (PPG)-mediated glycosidation approach for the synthesis of 2-deoxy-glycosides is reported. By employing ortho-nitrobenzyl carbonate (oNBC) as PPG, N,N-dimethylformamide (DMF)-modulated SPhosAuNTf2-promoted glycosidation with per-oNBC-protected 2-deoxy-glycosyl ynenoates afforded the 2-deoxy-glycosides with moderate to excellent α-selectivities presumably depending on the reactivities of the acceptor alcohols. Based on the PPG-mediated glycosidation approach, oligosaccharides with three to six oNBC groups were effectively achieved. The multiple oNBC groups in the 2-deoxy-glycosides were completely cleaved by irradiation at 365 nm, resulting in the desired 2-deoxy-glycosides in an efficient manner without affecting the common alkyne, alkene and azide functional groups and the traditional protecting groups on the aglycones. This article is protected by copyright. All rights reserved.Main observation and conclusion A green and efficient photolabile protecting group (PPG)-mediated glycosidation approach for the synthesis of 2-deoxy-glycosides is reported. By employing ortho-nitrobenzyl carbonate (oNBC) as PPG, N,N-dimethylformamide (DMF)-modulated SPhosAuNTf2-promoted glycosidation with per-oNBC-protected 2-deoxy-glycosyl ynenoates afforded the 2-deoxy-glycosides with moderate to excellent α-selectivities presumably depending on the reactivities of the acceptor alcohols. Based on the PPG-mediated glycosidation approach, oligosaccharides with three to six oNBC groups were effectively achieved. The multiple oNBC groups in the 2-deoxy-glycosides were completely cleaved by irradiation at 365 nm, resulting in the desired 2-deoxy-glycosides in an efficient manner without affecting the common alkyne, alkene and azide functional groups and the traditional protecting groups on the aglycones. This article is protected by copyright. All rights reserved.


