Citation:
Date Published:
FEBAbstract:
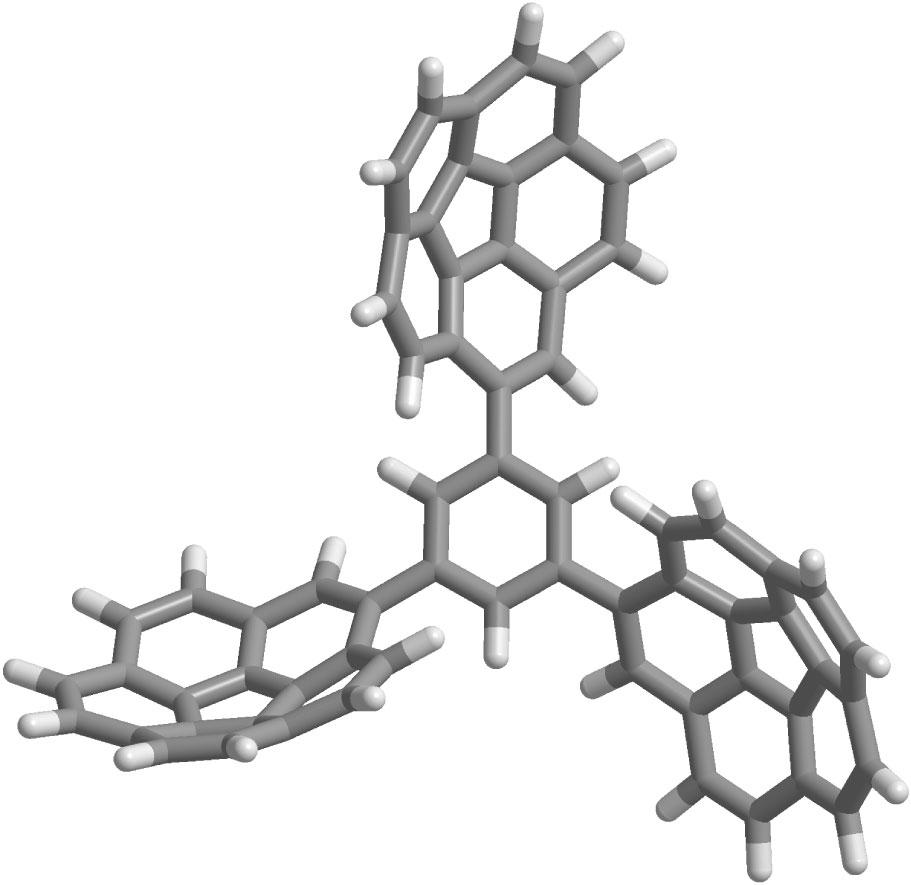
Oligocorannulenes are polyarenes composed of several corannulene units that are covalently linked. Their behavior arises both from the diverse properties of each corannulenyl unit and from the interactions between them. In this paper, we present the synthesis, stereochemistry, reduction, and self-assembly properties of a novel type of oligocorannulene: branched 1,3,5-tricorannulenylbenzene. Several stereodynamical elements combine to give rich stereochemistry: bowl-to-bowl inversion, rotation about the aryl-aryl single bonds, and residual stereoisomerism of molecular propellers. Reduction with lithium metal yields an intermediate hexaanion and ultimately produces a highly charged dodecaanion. Self-diffusion NMR demonstrates that the dodecaanion undergoes supramolecular dimerization through charged polyarene stacking, wherein two molecules are linked at all three contact points. The preference for dimerization over dendrimerization is attributed to an entropic effect. The dimer is found to undergo complex structural dynamics, as well as ion-pairing dynamics, as revealed by variable-temperature 1H- and 7Li-NMR. Copyright (C) 2012 John Wiley & Sons, Ltd.