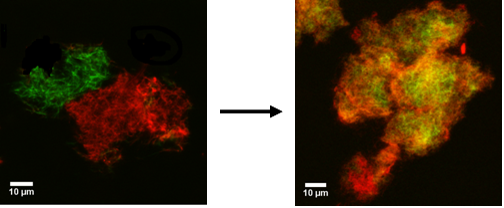
Amyloid beta (A\(\beta\)) 1-40, associated with neurodegenerative diseases such as Alzheimer's, tends to aggregate into fibrils with an extended β-sheet structure that accumulate outside neurons. The evolved fibrils have a very stable structure owing to their hydrophobic interactions and hydrogen bonds. We developed a facile way for studying dynamic processes in molecular aggregates composed of labeled A\(\beta\) (1-40) peptides. We used peptides labeled with either 5-Carboxytetramethylrhodamine (TAMRA) or Fluorescein-isothiocyanate (FITC). The labeled peptides were mixed after separate fibrillization, and the dynamic changes in the structure of the fibrils were imaged using confocal microscopy. In addition, fluorescence resonance energy transfer (FRET) measurements showed that Aβ (1-40) peptide molecules detached and reattached to the fibrils in a biological relevant time scale (days). With time, the two peptides mixed at the molecular level. This process was concentration dependent and occured primarily in the external parts of the aggregates with a half time of between 9 and 15 days. This study showed that the combination of confocal microscopy and FRET analysis is a facile method for studying dynamic processes in supramolecular aggregates. This work provides new insight into the dynamic nature of molecular aggregates and means for probing their nature and kinetics. This approach may be applied to a large variety of peptides and proteins and can form a basis for developing high throughput screening methods for investigating the action of various drugs on such aggregates (PCCP 2011). We have also investigated the effect of tachykinin neuropeptides on the aggregation process of Aβ(1-40). Our results suggest that there may be a specific interaction between tachykinins and Aβ(1-40) that allows them to coassemble. This effect may explain the reduction of Aβ(1-40) neurotoxicity in cells treated with tachykinins (BBRC 2011).