Virus particles are a remarkable example of self-assembled nanobiomaterial-based machines. Viruses encapsulate and release genetic materials at different points of their life cycle, at which they are exposed to a wide range of pH values. To assemble and disassemble, viruses should be both stable and dynamic. The interactions between the capsid and its genetic cargo are therefore crucial for understanding the function of viruses, for designing viral-based nanotechnology platforms and functional nanobiomaterials, or for sanitizing and disinfecting surfaces.
As we show in our paper, pH can dramatically affect the stability and infectivity of wild-type Simian Virus 40 (wt SV40), a member of the polyomavirus family. wtSV40 is a fascinating virus because it has a minichromosome, containing about 20 nucleosomes and 5.2 kb circular DNA, which is highly dynamic and hence difficult to study.
Using time-resolved cryo-TEM and state-of-the-art synchrotron time-resolved solution X-ray scattering, together with our novel X-ray scattering data analysis tools, developed in our lab in the past decade, we unraveled the disassembly reaction mechanism of wtSV40, triggered by a pH jump, at high temporal and structural resolutions. The pathway discovered in our paper, and shown in the illustration below, is likely to be similar in other members of the polyomavirus family, and perhaps in other viruses.
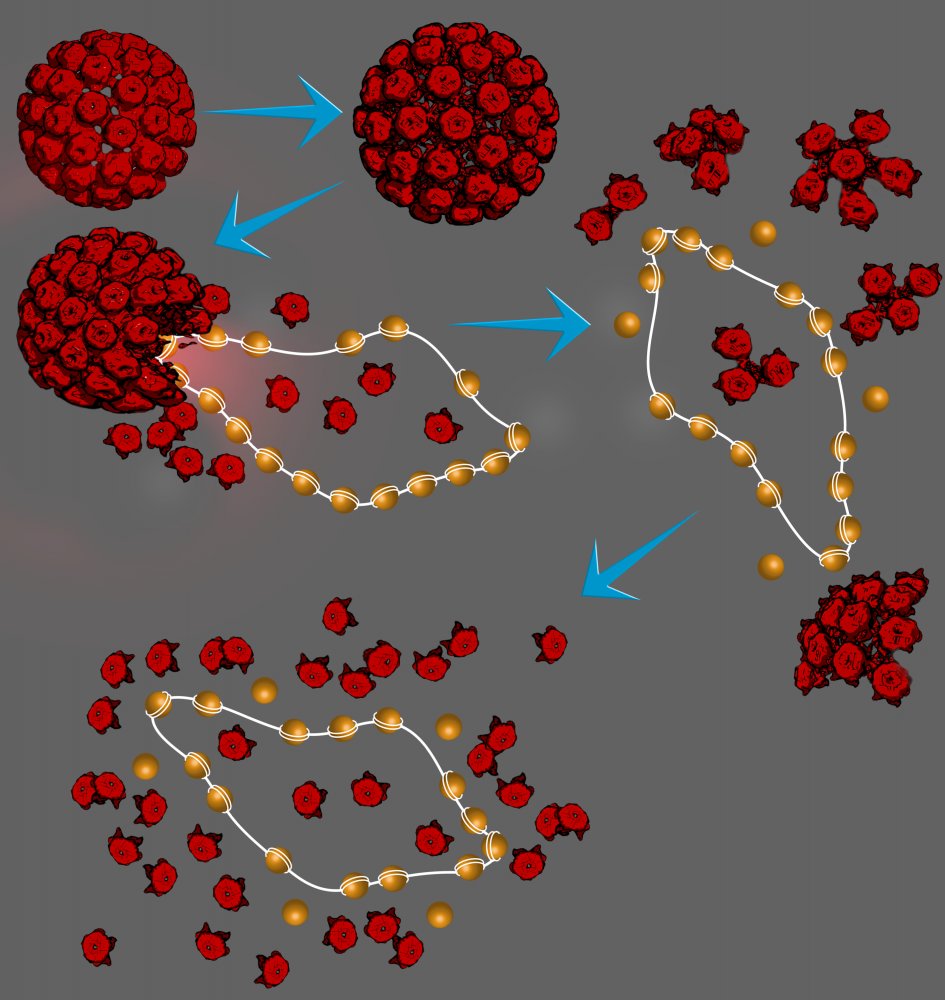
We have also showed that the virus has a wide pH stability and infectivity range, making it very attractive nanobiomaterial for various bionanotechnological applications and delivery systems.
Our study also provides insight into possible approaches for designing antiviral drugs and directing, tuning, and controlling the structure and properties of viruses or virus-like nanoparticle assemblies, which can serve as functional nanobiomaterials for bionanotechnological applications.
The novel approach developed in this study could be used for investigating other complex self-assembled nanobiomaterials.

