Citation:
Abstract:
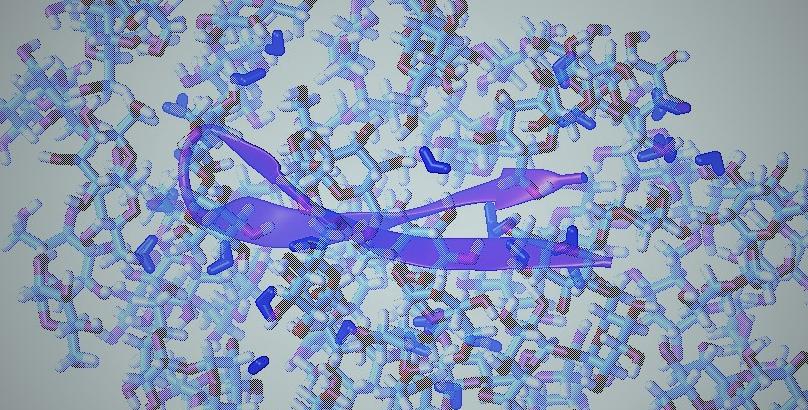
From stem cell freeze-drying to organ storage, considerable recent efforts have been directed toward the development of new preservation technologies. A prominent protein stabilizing strategy involves vitrification in glassy matrices, most notably those formed of sugars such as the biologically relevant preservative trehalose. Here, we compare the folding thermodynamics of a model miniprotein in solution and in the glassy state of the sugars trehalose and glucose. Using synchrotron radiation circular dichroism (SRCD), we find that the same native structure persists in solution and glass. However, upon transition to the glass, a completely different, conformationally restricted unfolded state replaces the disordered denatured state found in solution, potentially inhibiting misfolding. Concomitantly, a large exothermic contribution is observed in glass, exposing the stabilizing effect of interactions with the sugar matrix on the native state. Our results shed light on the mechanism of protein stabilization in sugar glass and should aid in future preservation technologies.
Notes: