Citation:
Abstract:
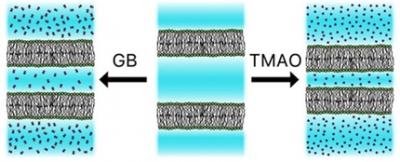
Ubiquitous in the cellular milieu, small organic compounds termed osmolytes help to regulate the response to environmental stress. Elasmobranchii, such as sharks, accumulate high concentrations of several osmolytes, most notably urea, trimethylamine N‐oxide (TMAO), and glycine betaine (GB). These three compounds are used to osmoregulate the organism's body fluids, so that they counteract seawater salinity, as well as the destabilizing effects of hydrostatic pressure on cells and their molecular components. Herein we focus on glycine betaine and show how it modifies interactions between lipid membranes. We find that the addition of GB exerts an apparent attractive force that draws neighboring membranes toward one another. We show that, at the molecular level, this apparent attraction between membranes in the presence of GB is due to its preferential exclusion from the space between adjacent membranes, which thereby exerts an osmotic pressure that brings membranes closer together. This action is similar to the one we have previously reported for TMAO. However, we find that the net effect of GB is significantly smaller than that of TMAO, because GB concomitantly significantly weakens van der Waals attractions between membranes by changing the dielectric properties of the intervening solution. Finally, we show how GB acts in combination with urea. At high total concentrations and over a wide range of GB‐to‐urea ratios, urea counteracts the effect of GB, so that the equilibrium separation between membranes is close to their values in pure water. This finding supports the prevailing idea that mixtures of several osmolytes can achieve minimal net impact on biomacromolecular stability while also counteracting osmotic stress.
Notes: