This is the page of the ERC project "PhotoMutant"
To understand the spectral tuning mechanism we have studied Slr1393g3, a member of the Cyanobacteriochrome photoreceptor protein family. This protein switches from a red- to a green-absorbing form upon light absorption. We have identified the mechanism of this color tuning by application of hybrid quantum mechanics/molecular mechanics (QM/MM) simulation method. This protein, related to classical phytochromes, carries the open-chain tetrapyrrole chromophore phycocyanobilin. Our calculations have revealed that the effective conjugation length in the chromophore becomes shorter upon conversion from the red to the green form. This is related to the planarity of the entire chromophore. A large distortion is found for the terminal pyrrole rings A and D, however, the D ring contributes stronger to the photoproduct tuning, despite a larger change in the twist of the A ring. Our findings implicate that the D ring twist can be exploited to regulate absorption of the photoproduct. Hence, mutations that affect the D ring twist can lead to rational tuning of the photoproduct absorption that allows tailoring of cyanobacteriochromes for biotechnological applications such as optogenetics and bioimaging.
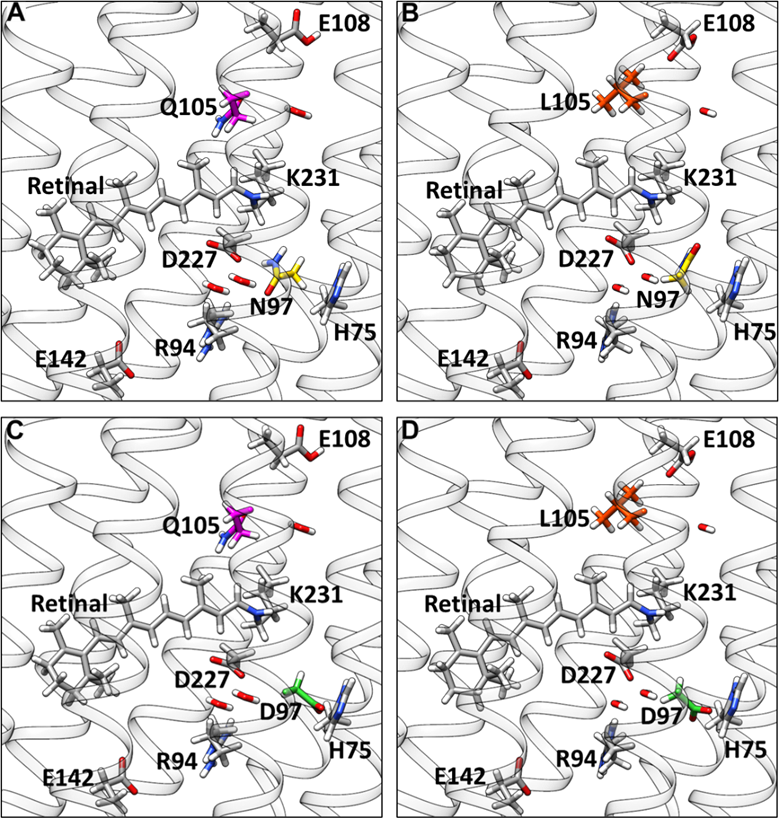
We also studied the spectral tuning mechanism in the blue proteorhodopsin (BPR), because the crystal structure of this protein was available and provided the basis for our computer simulations. The investigation included 4 variants of the BPR which included the wild type, the Q105L mutant, the D97N as well as the double mutant D97N/Q105L (Fig. 1). First we calculated UV/Vis spectra of this 4 variants using QM/MM MD sampling. The results were in qualitative agreement with the experimental data. This gave us confidence in the quality of the simulations. We then analyzed the structural parameters and found that the retinal in wild type BPR has the highest bond order alternation, which means that the difference between the single and double bonds is most pronounced for this protein. Following this finding we looked at the spectra of retinal without the protein and found them to be nearly identical. We concluded that the direct electrostatic effect of the protein is causing the shift. But this seemed to be in conflict with the fact that both L and Q are neutral. However, is seems that there is a steric repulsion by Q105 which makes the chromophore more twisted. And this twisted retinal is more sensitive to the color tuning by Q105. This is confirmed by the fact that the spectral shift between D97N and D97N/Q105L is smaller. Hence, we deduced that Q105 induces a twist in the retinal and D97 counterion is polarizing retinal to get an enhanced shift. It means that both Q105 and D97 are required to have a spectral shift.
Further we have studied the primary photochemical reaction of the green-absorbing Proteorhodopsin by the same hybrid QM/MM approach. The simulations are based on a homology model derived from the blue-absorbing Proteorhodopsin crystal structure. The geometry of retinal and the surrounding sidechains in the protein binding pocket were optimized using the QM/MM method. Starting from this geometry the isomerization was studied with a relaxed scan along the C13=C14 dihedral. It revealed an “aborted bicycle pedal” mechanism of isomerization that was originally proposed by Warshel for bovine rhodopsin and bacteriorhodopsin. However, the isomerization involved the concerted rotation about C13=C14 and C15=N, with the latter being highly tiwsted but not isomerized. Further, the simulation showed an increased steric interaction between the hydrogen at the C14 of the isomerizing bond and the hydroxyl group at the neighbouring tyrosine 200. In addition, we have simulated a nonadiabatic trajectory which showed the timing of the isomerization. In the first 20 fs upon excitation the order of the conjugated double and single bonds is inverted, consecutively the C13=C14 rotation is activated for 200 fs until the S1-S0 transition is detected. However, the isomerization is reverted due to the specific interaction with the tyrosine as observed along the relaxed scan calculation. Our simulations indicate that the retinal - tyrosine 200 interaction plays an important role in the outcome of the photoisomerization.

