Research in my laboratory focuses on the role of inflammatory processes, in general, and brain microglia cells, in particular, in regulation of cognition, emotion, neural plasticity and neurogenesis. Specifically, we focus on the following three inter-related projects:
1) The role of microglia and inflammatory cytokines in pathophysiology of major depression.
Research in Yirmiya's laboratory during the early 1990' provided the first experimental evidence for a relationship between immune activation and major depression. In parallel studies, the Yirmiya's laboratory developed some of the first controlled and prospective experimental models of disease-induced psychological changes in humans, demonstrating that immune challenges, such as endotoxin administration, rubella vaccination or minor surgery, induce cytokine-mediated depressed mood and cognitive impairments. Together, these studies directly contributed to the formation of novel conceptualizations and the development of clinically effective treatments for inflammation-associated major depression.
In recent years, research in the Yirmiya's laboratory was mainly focused on the specific role of brain microglia in depression. We found that exposure of mice to chronic unpredictable psychological stress, which is one of the leading causes of depression in humans, leads to dynamic alterations in the structure and function of microglia cells. Furthermore, treatment of "depressed-like" mice with compounds that modulate microglial proliferation and activation, reversed the depressive-like behavior and dramatically increased hippocampal neurogenesis (Kriesel et al., Molecular Psychiatry, 2014; see image below).


These findings have important theoretical and clinical implications. According to our new theory, either activation or decline of microglia can lead to depression, and therefore it is clear that this disease cannot be treated uniformly (by the same class of drugs, which is the current psychiatric practice). Rather, a personalized medical approach should be adopted, in which the status of microglia in the individual patient should be first established, and based on this initial measurement treatment with drugs that either inhibit the over-active microglia (e.g., minocycline) or stimulate the suppressed microglia (e.g., M-CSF or GM-CSF) should be employed (Yirmiya et al., TiNS, 2015; see image below).

Deviations from microglial homeostasis induce depression: Therapeutic implications. Normal physiological functioning of microglia is essential for normal mood levels. Accordingly, extreme deviations from the normal microglial activation status may be the cause of some forms of depression. Such deviations include microglial activation associated with peripheral and central infections, autoimmune and neurodegenerative diseases, trauma, stroke, and acute psychological stress. Microglial decline, senescence, dystrophy, and suppression, which sometimes occur following long periods of microglial activation, e.g., due to aging, Alzheimer's disease, or certain chronic stress regimens, also represent a deviation from microglial homeostasis. The alterations from the normal quiescent microglial morphology, represented by the ramified microglia in the center of the figure (green = genetic labeling of microglia with GFP, blue = DAPI nuclear staining) are exemplified by the activated microglia from the brain of an acutely stressed mouse and the dystrophic microglia from the brain of a mouse exposed to CUS (right and left sides of the figure, respectively). In contrast with the consequences of extreme microglial states, mild microglial proliferation and activation, which can be induced by environmental enrichment or physical activity, is associated with resilience to depression. According to this model, depression should be treated by a personalized medical approach based on the basal (pre-treatment) microglial status (declined vs. fully activated) of the individual depressed patient, using microglia stimulating or suppressing drugs, respectively.
A recent review (Yirmiya, Brain, Behavaior and Immunity, 2024) summarizes the evidence for the hypothesis that dysregulation of inflammatory processes plays a critical role in the pathophysiology of depression. This review traces the evolution of research supporting this link, discussing key findings from several major investigative fronts: Alterations in inflammatory markers associated with depression; Mood changes following the exogenous administration of inflammatory challenges; The anti-inflammatory properties of traditional antidepressants and the promising antidepressant effects of anti-inflammatory drugs. Additionally, it explores how inflammatory processes interact with specific brain regions and neurochemical systems to drive depressive pathology. A thorough analysis of the 100 most-cited experimental studies on the topic ensures a comprehensive, transparent and unbiased collection of references. This methodological approach offers a panoramic view of the inflammation-depression nexus, shedding light on the complexity of its mechanisms and their connections to psychiatric categorizations, symptoms, demographics, and life events. Synthesizing insights from this extensive research, the review presents an integrative model of the biological foundations of inflammation-associated depression (see the figure below). It posits that we have reached a critical juncture where the translation of this knowledge into personalized immunomodulatory treatments for depression is not just possible, but imperative.
2) The role of microglia and inflammatory cytokines in neurobehavioral processes, including memory consolidation, neural plasticity, and neurogenesis.
Studies is my laboratory over the past two decades discovered that the immune system plays a central role in modulating learning, memory and neural plasticity. Under normal quiescent conditions, immune mechanisms are activated by environmental/psychological stimuli and positively regulate the remodeling of neural circuits, promoting memory consolidation, hippocampal long-term potentiation (LTP) and neurogenesis. These beneficial effects of the immune system are mediated by complex interactions among brain cells with immune functions (particularly microglia and astrocytes), peripheral immune cells (particularly T cells and macrophages), neurons, and neural precursor cells. These interactions involve the responsiveness of non-neuronal cells to classical neurotransmitters (e.g., glutamate and monoamines) and hormones (e.g., glucocorticoids), as well as the secretion and responsiveness of neurons and glia to low levels of inflammatory cytokines, such as interleukin (IL)-1, IL-4, and TNFα, as well as other mediators, such as prostaglandins and neurotrophins (see left panel of the image below). In conditions under which the immune system is strongly activated by infection or injury, as well as by severe or chronic stressful conditions, glia and other brain immune cells change their morphology and functioning and secrete high levels of pro-inflammatory cytokines and prostaglandins. The production of these inflammatory mediators disrupt the delicate balance needed for the neurophysiological actions of immune processes and produces direct detrimental effects on memory, neural plasticity and neurogenesis. These effects are mediated by inflammation-induced neuronal hyper-excitability and adrenocortical stimulation, followed by reduced production of neurotrophins and other plasticity-related molecules, facilitating many forms of neuropathology associated with normal aging as well as neurodegenerative and neuropsychiatric diseases (see right panel of the image below).
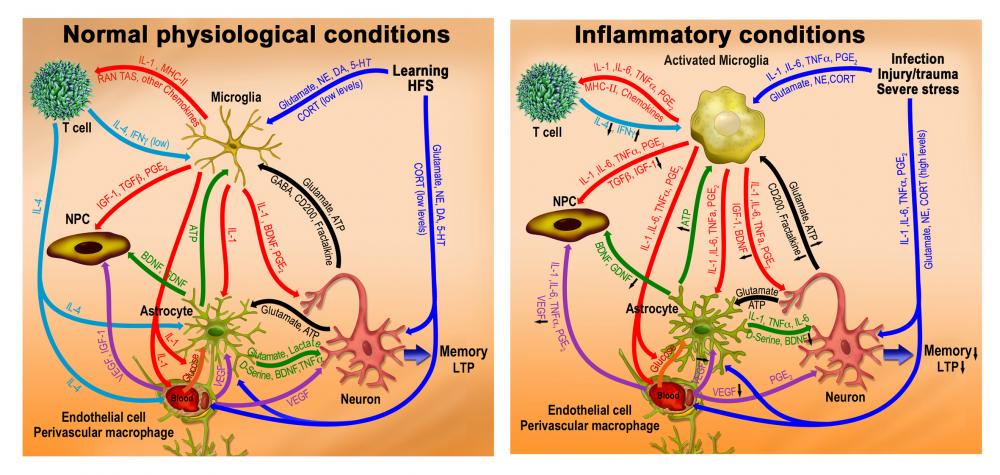
Immune modulation of neuro-behavioral functioning: (Left panel) During normal quiescent conditions, the immune system supports and boosts brain activity, promoting learning, memory, LTP and neurogenesis. These beneficial effects depend on inflammatory cytokines-mediated interactions among brain immune cells, particularly microglia, neurons and neural precursor cells. (Right panel) During infection, injury or severe/chronic stress, the brain "cytokine storm" disrupts the delicate balance needed for the normal neurophysiological and cognitive actions of immune processes. The resultant impairments in neurobehavioral plasticity involve neuronal hyper-excitability followed by homeostatic adrenocortical stimulation, reductions in plasticity-related molecules, cognitive disturbances and depression.
3) Neuropsychosteology
Based on the template of psychoneuroimmunology, my laboratory, in collaboration with Professor Itai Bab, took part in developing a new discipline that we termed "Neuropsychosteology" (Yirmiya et. al., PNAS 2006). This discipline focuses on the bi-directional interactions between the nervous system and bone. We examined many aspects of these interactions, particularly focusing on the detrimental effects of chronic stress and depression on bone mineral density and the role of the sympathetic nervous system in mediating these effects (see the image below for example).

Depression-induced structural impairment of the skeleton in mice exposed to chronic mild stress (CMS) for 4 weeks or left untreated (UT); 3D μCT skeletal analysis. Trabecular bone volume density (BV/TV) is expressed as percent volume of trabecular network of total reference volume.

