Citation:
Abstract:
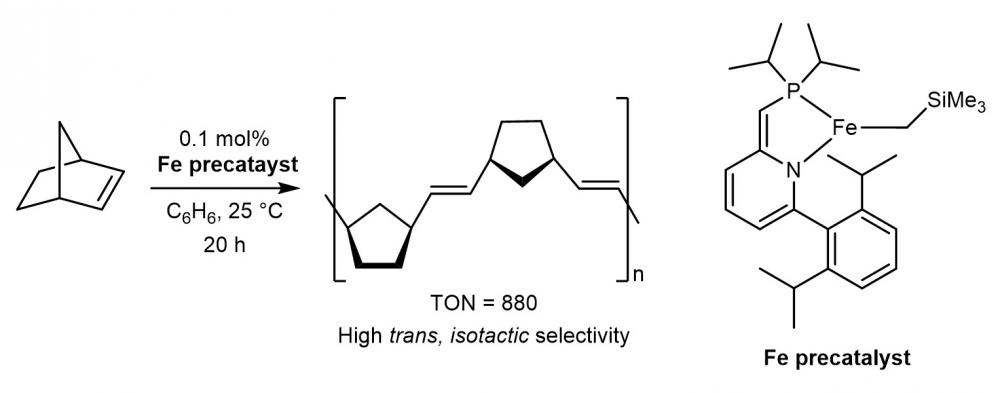
The olefin metathesis reaction is among the most widely applicable catalytic reactions for carbon–carbon double bond formation. Currently, Mo– and Ru–carbene catalysts are the most common choices for this reaction. It has been suggested that an iron-based catalyst would be a desirable economical and biocompatible substitute of the Ru catalysts; however, practical solutions in this regard are still lacking. Here, we report the discovery and mechanistic studies of three-coordinate iron(II) catalysts for ring-opening metathesis polymerization of olefins. Remarkably, their reactivity enabled the formation of polynorbornene with stereoregularity and high molecular weight (>107 g mol–1). The polymerization in the presence of styrene revealed cross metathesis reactivity with iron catalysts. Mechanistic studies suggest the possible role of metal–ligand cooperation in formation of the productive catalyst. This work opens the door to the development of iron complexes that can be economical and biocompatible catalysts for olefin metathesis reactions.