In collaboration with Zvonimir Dogic, we are using bacterial flagellum to assemble rigid filaments. Each flagellum has the shape of a hollow cylinder with an outer diameter of about 23 nm. It is comprised of 11 protofilaments staggered in a helical arrangement. In turn, each protofilament is assembled from a linear row of flagellin monomers. Flagellin monomers can exist in two distinct conformational states, denoted by L and R form, that have a slightly different size. Hundreds of flagellin monomers within each protofilament cooperatively switch between these two states, a conformational transition that leads to a net change in the protofilament length. If all the protofilaments within one flagellum have the same conformational state the filament assumes a straight shape. However, in many cases each flagellum contains a mixture of L and R protofilaments. A different length of these protofilaments result in a packing frustration that is resolved by the formation of a well-defined helical structure. The number of protofilaments (11) dictates 10 distinct helical shapes and two straight filament arrangements in which all protofilaments assume either L or R configuration. Importantly flagella can switch between different polymorphic states in response to changing external stimuli, such as ionic strength, solvent composition, pH and temperature as well as application of torque. Furthermore, point mutations in the flagellin amino-acid sequence also affect the conformation of protofilaments and thus the overall helical structure. Flagellin mutants have been isolated in which all of the protofilaments assume the same L or R conformational state, resulting in assembly of straight flagella. Copolymerizing a mixture of these two flagellin monomers results in the assembly of filaments, whose helical state is determined by the ratio of the L and R monomer types. This strategy allows for in-vitro assembly of filaments with all 12 possible shapes.
We have used flagellin monomers with point mutations in their amino acid sequence, which have been isolated from S. Typhimurium strains. Using exiting filaments as seeds for subsequent polymerization reactions control the filament shape. Controlling the ratio of seeds to flagellin monomers determines the length of polymerized filaments. Using this strategy we have ssembled filaments whose average length ranges anywhere from a few up to about 50 μm.
Using small-angle x-ray scattering and osmotic stress methods, we investigated the structure of SJW1660 flagellar filaments as well as the intermolecular forces that govern their assembly into dense hexagonal bundles (Biophys. J. 2017). The scattering data were fitted to models, which took into account the atomic structure of the flagellin subunits.
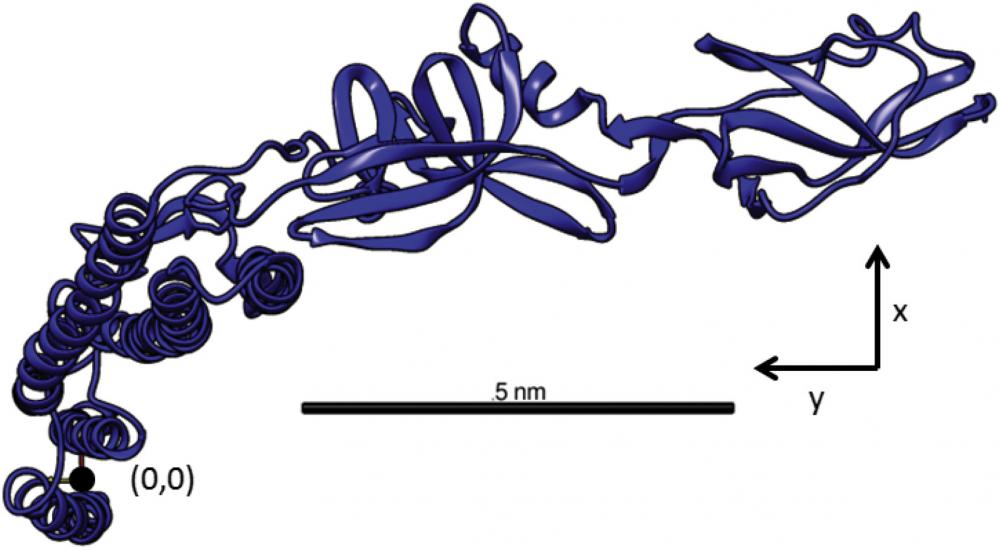
The analysis revealed the exact helical arrangement and the super-helical twist of the flagellin subunits within the filaments.
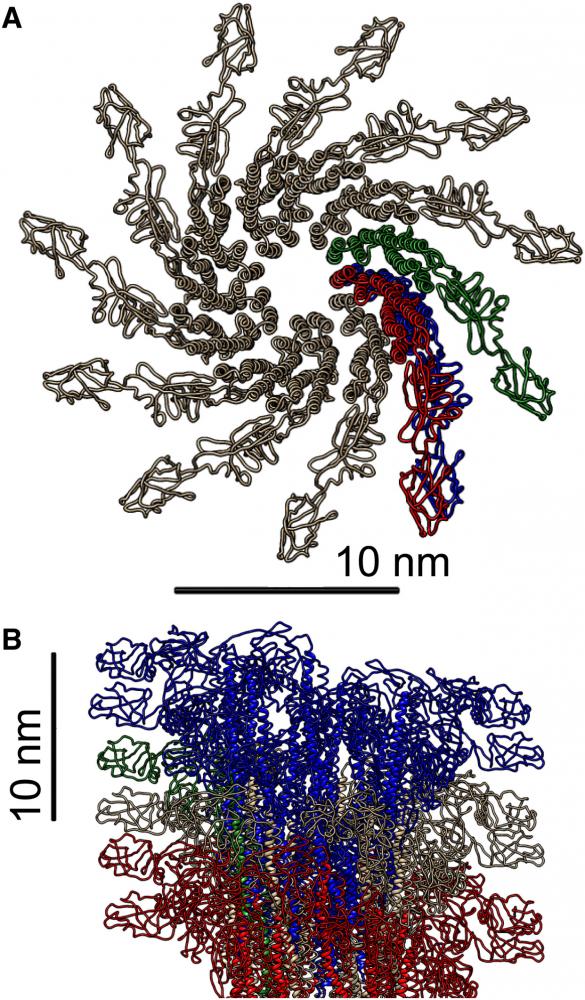
Under osmotic stress, the filaments formed two-dimensional hexagonal bundles.
Monte Carlo simulations and continuum theories were used to analyze the effect of thermal fluctuations and to fit the scattering data from hexagonal arrays, revealing how the bundle bulk modulus and the deflection length of filaments in the bundles depend on the applied osmotic stress.
Scattering data from aligned flagellar bundles confirmed the theoretically predicated structure-factor scattering peak line shape.
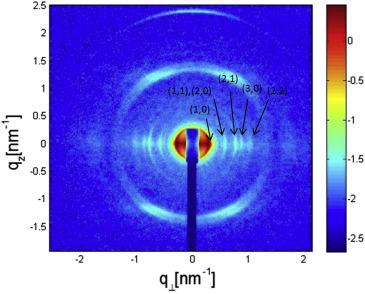
Quantitative analysis of the measured equation of state of the bundles revealed the contributions of electrostatic, hydration, and elastic interactions to the intermolecular forces associated with bundling of straight semi-flexible flagellar filaments (Biophys. J. 2017).

