Microtubules (MTs) are 25 nm protein nanotubes with walls comprised of assembled protofilaments built from \(\alpha\beta\) heterodimeric tubulin.
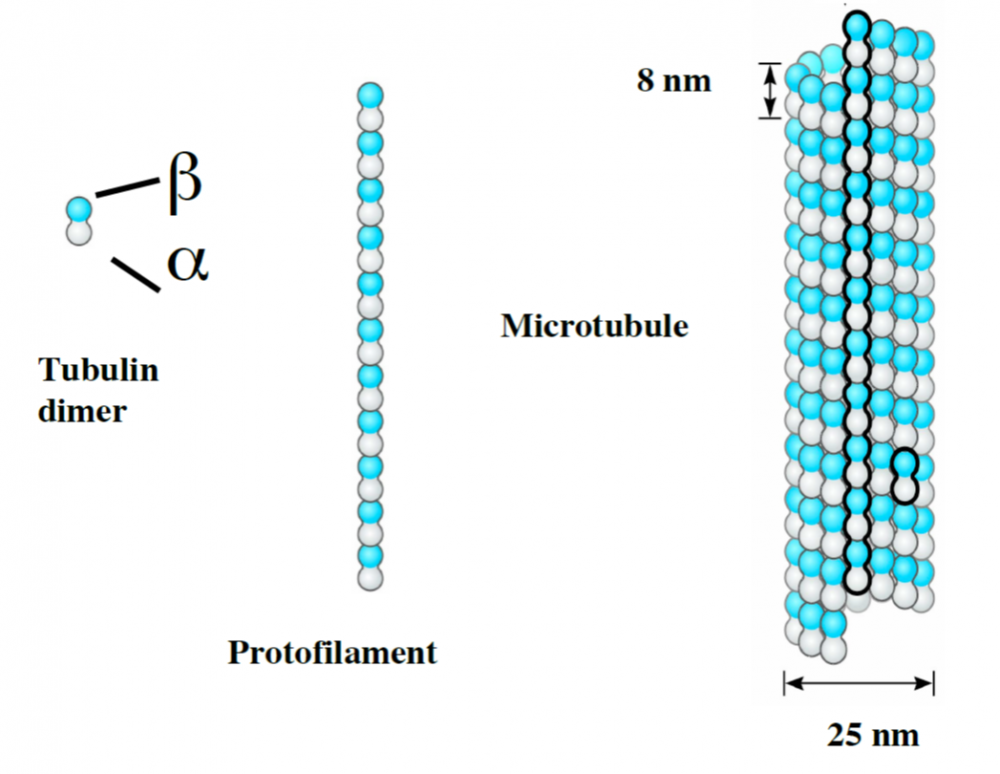
MT are dynamic structures exhibiting complex behavior. Disruption of normal MT dynamics leads to neuronal process degeneration and neuronal cell death. MT assembly requires GTP-bound-\(\alpha\beta\)-tubulin, and GTP hydrolysis by \(\beta\)-tubulin (into GDP) is required to generate dynamic MTs. Tubulin nucleation and MT assembly are highly abundant processes in cytoskeleton biology. A fair amount of skill is needed to study tubulin and MTs in their natural dynamic state owing to the small size of tubulin dimers and the dynamic character of their self-assembled structures.
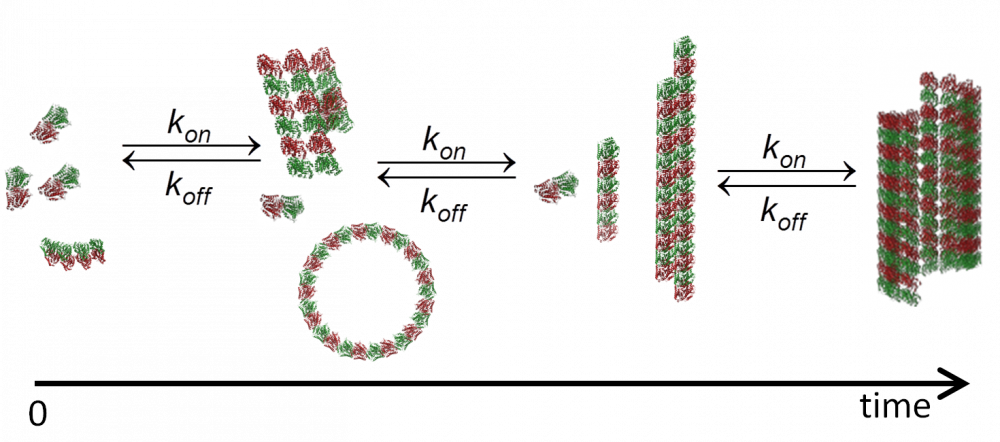
We purify tubulin from porcine brains (as explained in Biochemistry 2018) and use them without any further modifications or additives (check our tubulin concentration measurement protocol). We have developed experimental protocols to study the high-resolution structure and dynamics of tubulin and MT in their natural state (J. Phys. Chem. 2017) and in the presence of drugs (Phys. Rev. Lett. 2004, Biophys. J 2005, 2007), ions (PNAS 2004, Faraday Discuss. 2013, Nature Materails 2014), and/or the microtubule associated protein (MAP) tau (Biophys. J 2009, PNAS 2015). Tubulin and MT are important targets for several clinically used drugs, including colchicine, used for gout, and the anticancer drugs paclitaxel (taxol), vincristine, and vinblastine. The stability of MT, its assembly, and disassembly processes are tightly regulated by MAPs. Tau is a particularly important MAP because aberrant tau function has been implicated in numerous diseases. Tau is a highly soluble protein but in its misfolded state it may aggregate. Diseases caused by misfolded tau are called tauopathies. Pure tauopathies (diseases that only involve abnormal tau) include Pick’s disease, progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and argyrophilic grain disease. A combination of abnormal tau and other proteins (tubulin, for example) play a destructive role in Alzheimer’s disease, chronic traumatic encephalopathy (associated with head trauma), Niemann-Pick-C, Guam-ALS-Parkinson’s-dementia, aluminum toxicity and post-encephalitic Parkinson’s disease.

