Entropic attraction condenses like-charged interfaces composed of self-assembled molecules.
Like-charged solid interfaces repel and separate from one another as much as possible. Charged interfaces composed of self-assembled charged-molecules such as lipids or proteins are ubiquitous. We showed that although charged lipid-membranes are sufficiently rigid, in order to swell as much as possible, they markedly deviate from the behavior of typical like-charged solids when diluted below a critical concentration of about 15 wt%. Unexpectedly, charged membranes swell into lamellar structures with spacing that is up to four times shorter than the layers should assume (if they are filling the entire available space). This process is reversible with respect to changing the lipid concentration. While the repulsion between charged interfaces increases with temperature, like-charged membranes remarkably condense with increasing temperature. This effect is also reversible. To better understand the effect we carefully analyzed (using both SAXS and WAXS) the effect of temperature on the structure of the membrane (J. Phys. Chem. B 2012). Although the membranes thin with temperature they do not thin enough to explain the data.
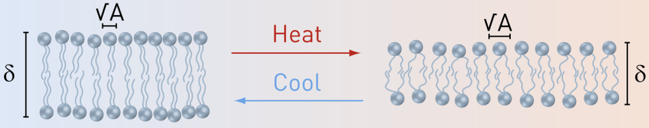
Our findings hold for a wide range of conditions including varying membrane charge density, bending rigidity, salt concentration, and conditions of typical living systems. We attribute the limited swelling and condensation of the net repulsive interfaces to their self-assembled character. Unlike solids, membranes can rearrange to gain an effective entropic attraction, which increases with temperature and compensates for the work required for condensing the bilayers. We also showed that dipolar interfaces that become charged under certain conditions and settings of typical living systems behave in a similar way.
The findings provide new insight into the thermodynamics and self-organization of like-charged interfaces and dipolar interfaces composed of self-assembled molecules. Because most water soluble macromolecules can self-assemble and exhibit charged or dipolar interfaces our analysis provides a comprehensive and unique insight into the self-organization of biomaterials and supramolecular assemblies that are widely found in nature (Langmuir 2011; 2012).


